Malaria & Schistosomiasis
Prevalence of glucose-6-phosphate dehydrogenase (G6PDH) deficiency coincides with that of malaria exhibiting a distribution with 400 million people being affected globally; this supports the socalled malaria protection hypothesis. Mediterranean populations with G6PDH deficiency provide a natural biomimetic knock-out version of human glutathione reductase (hGR) because G6PDH is the main provider of NADPH for GR in the erythrocyte. The deficiency is not lethal for humans but prevents severe attack of malaria since the oxidative stress released in the erythrocytes renders the milieu hostile for Plasmodium which cannot pursue its growth cycle efficiently. The aim of our present proposal is to mimic the G6PDH deficiency by developing redox-active GR inhibitors.
In the team different types of inhibitors (reversible, irreversible) were early designed in the 1,4- naphthoquinone (NQ) series to evaluate the impact of each inhibition mode on the growth of the parasites: uncompetitive or catalytic inhibitors and fluorine-based suicide-substrates, dual prodrugs based on disulfide reductases inhibitors. Our work on targeting redox equilibria of malarial parasites propagating in red blood cells has led to the selection of a series of 3-benzyl-1,4-naphthoquinones (benzylNQ) which are active at nM concentrations against the human pathogen P. falciparum in culture and against P. berghei in infected mice. The series is active in vitro in the low nM range against 12 strains of P. falciparum expressing different degrees of resistance to chloroquine and quinine and in vivo in murine models (per os). With respect to safety, the compounds do not trigger hemolysis or other signs of toxicity in mice. Concerning the antimalarial mode of action, we proposed that the lead benzylNQ are initially oxidized at the benzylic chain to benzoyl-1,4-naphthoquinones (benzoylNQ) in a heme-catalyzed reaction within the digestive acidic vesicles of the parasite (Figure 1).
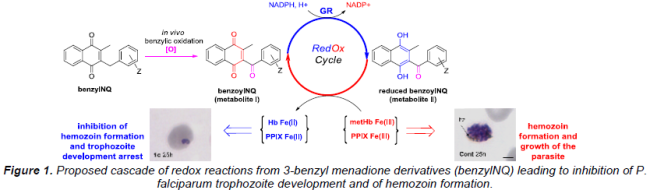
The major putative benzoyl metabolites were then found to function as redox cyclers: (i) in their oxidized form, the benzoyl metabolites are reduced by NADPH in glutathione reductase-catalyzed reactions within the cytosols of infected red blood cells; (ii) in their reduced forms, these benzoyl metabolites can convert methemoglobin (metHb), the major nutrient of the parasite, to indigestible hemoglobin (Hb). Pre-reduction of the benzoylNQ by the GR/NADPH system was found to be a critical step and only the hydro-benzoylNQ are the key species capable to reduce metHb. Besides, studies on a fluorinated suicide-substrate indicate as well that the glutathione reductase-catalyzed bioactivation of naphthoquinones is essential for the observed antimalarial activity. On the basis of a physico-biochemical study the benzoylNQ possess a high affinity (KD in the µM range) for hematin: Π-Π dimer comparable to those measured for the major antimalarial drugs. A direct correlation between ability to reduce metHb and to inhibit hemozoin (hz) polymerization was evidenced in parasites (photos in Figure 1).
In conclusion, the antimalarial naphthoquinones are suggested to perturb the major redox equilibria of the targeted infected red blood cells which might be removed by macrophages in vivo. This results in development arrest and death of the malaria parasite at the trophozoite stage. Thus, the antimalarial benzylNQ might act as redox-active electron acceptors – through redox drug bioactivation – being cycled in and out the food vacuole in the parasites, likely through iron complexation.
Trypanosomiasis and Leishmaniasis 
As part of our efforts to discover new agents to cure trypanosomiasis and leishmaniasis, we developed two chemical series that can drastically affect the redox equilibria of these parasites. Both series possess two electrophilic sites, and were proposed to undergo Michael addition with trypanothione, the major dithiol of kinetoplastids. This thiol is regenerated by the NADPH-dependent trypanothione reductase which has been identified as a drug target for the search of new antikinetoplastidal agents. Both series are highly active against parasites in cultures but exhibited a high toxicity againts human cells (IC50 hMRC5 = 1.5 µM) and in T. brucei-infected mice.
In order to reduce this toxicity while maintaining the trypanocidal activity, we synthesized dissymmetric divinylketones and prodrugs. A fine tuning of the electrophylicity of both centers might allow to modulate the toxicity. As the synthesis of these dissymmetric divinylketones is not easy through the classical synthetic pathway (Claisen reaction), an optimized tandem Sonogashira coupling-isomerisation methodology under microwave irradiation was established in one pot, in collaboration with Prof. T. J. J. Müller, Düsseldorf (Germany). More than 15 compounds substituted with pyridines, pyrimidines, and fluoroaromatics were prepared.