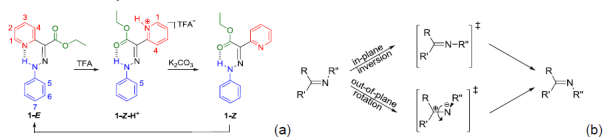
Isomerization Mechanism in Hydrazone-Based Rotary Switches
The group of Prof. I. Aprahamian (Dartmouth College, Hanover, USA) has recently designed a potential platform of new rotary switches based on a hydrazone building block. These derivatives exist as the E isomer at equilibrium, and can be switched to the protonated Z configuration (Z-H+) by addition of acid. Protonation of the pyridine indeed induces a rotation around the hydrazone C=N double bond, leading to isomerization. Treating Z-H+ with base yields a mixture of E and "metastable" Z isomers. The latter thermally equilibrates to reinstate the initial isomer ratio. Isomerization around the C=N double bond (Scheme 1) is known to proceed via two different mechanisms: rotation (polar transition state) and inversion (non-polar transition state). We investigated the kinetics, the thermodynamics and the activation parameters of the switching process of two derivatives (phenyl or anthryl derivatives). We demonstrated that the Z<->E isomerization involves a highly organized polarized transition state and hence proceeds via a rotation mechanism. These molecular switches represent the initial examples of chemically controlled configurational rotary switches. Current efforts taking advantage of these new insights are under progress to better tune the performance of these hydrazone-based molecular switches.
Physico-Chemical Investigations of Stable and Inert 64Cu and 89Zr Chelators
Advances in the cyclotron production routes for 64Cu and 89Zr using low and high energy cyclotrons have led to their renewed interest as Positron Emission Tomography isotopes and radiolabels. 64Cu (t1/2 = 12.7 h) and 89Zr (t1/2 = 78.4 h) are characterized by t1/2 matching those of biological processes such as immunogenic reactions and are, consequently, attractive isotopes in the context of PET imaging and radioimmunotherapy. Their use, however, requires the development of new bifunctional chelators (BFCs) displaying strong, stable, rapid and selective complexation of both Cu(II) and Zr(IV) and containing an activated function for grafting them on biological materials. If numerous chelators have been designed to strongly chelate Cu(II) and, at a lesser extend, Zr(IV), the major drawbacks concern the slow formation kinetics and the weak kinetic inertness and selectivity. Besides, the Zr(IV) speciation chemistry is still a rather unexplored area. Prior to the near setting up (March 2012) of the 24 MeV cyclotron in Strasbourg-Cronenbourg, this ongoing project carried out in scientific collaboration with Dr. L.J. Charbonnière (IPHC, UMR 7178 CNRS/UdS, ECPM) is aimed at designing, developing and thoroughly studying new molecular tools with high potential as 64Cu and 89Zr chelators. Special attention is focused on the determination of their physico-chemical properties under physiological conditions. We recently demonstrated that L1, a non macrocyclic polyaminomethane-phosphonate ligand, rigidified by a central pyridyl unit, is an excellent Cu(II) chelator, rivaling with most of the functionalized cyclen and cyclam derivatives. By contrast with the other reported systems, L1 leads to very stable, selective and inert Cu(II) complexes. Besides these fundamental physico-chemical properties, fast Cu(II) speciation was also demonstrated.