|

|

|

|

|

|
Synthetic Strategies and original sugar mimetics
The molecular diversity/complexity of carbohydrates and their central role in biology challenge the imagination of both chemists and biologists. Recent major progress in glycobiology has provided evidence for the paramount importance of oligosaccharides and glycoconjugates in a number of fundamental processes such as intercellular recognition, viral infections or rheumatoid arthritis. However, despite these recent scientific breakthroughs, the full potential of glycobiology in therapeutic context is yet to be realized.
The carbohydrate themselves are rarely good drug candidates for several reasons including instability or difficult synthesis. Consequently, for many years, the vast potential of glycoscience to create therapeutics was almost unexplored by pharmaceutical companies. Glycomimetics, as structurally modified simple analogs of carbohydrates, hold the key to translate the discoveries of glycobiology into therapeutic applications as demonstrated very recently by pioneering examples (e. g. Tamiflu®). These small molecules are rationally designed to enhance or modulate the biological activity of the native carbohydrate structures and to function more like traditional drugs with the required bioavailability and pharmacokinetics.
Iminosugars are sugars in which the endocyclic oxygen is replaced by a basic nitrogen atom. This apparently simple substitution raises many synthetic challenges and opens the way to remarkable biological properties. As such, iminosugars form undoubtedly the most attractive class of carbohydrate mimics reported so far. Over the past decade, the pace of discoveries in the field of iminosugars has been breathtaking. Historically known as glycosidase inhibitors, the scope of their biological activity has been extended to the inhibition of numerous enzymes such as glycosyltransferases, glycogen phosphorylases, nucleoside-processing enzymes, UDP-Galp mutase and more recently metalloproteinases. As a consequence, iminosugars are now lead compounds for the treatment of an impressive variety of diseases including diabetes, cancers, viral infections, psoriasis and rare genetic diseases (lysosomal storage disorders and cystic fibrosis). The recent approval of Galafold™ as the first oral treatment for Fabry disease, a rare genetic disease, is a spectacular demonstration of the importance of iminosugars as medicines for unmet medical needs. In this context, the development of rapid and general access to original iminosugars is more than ever highly needed.
Selected examples
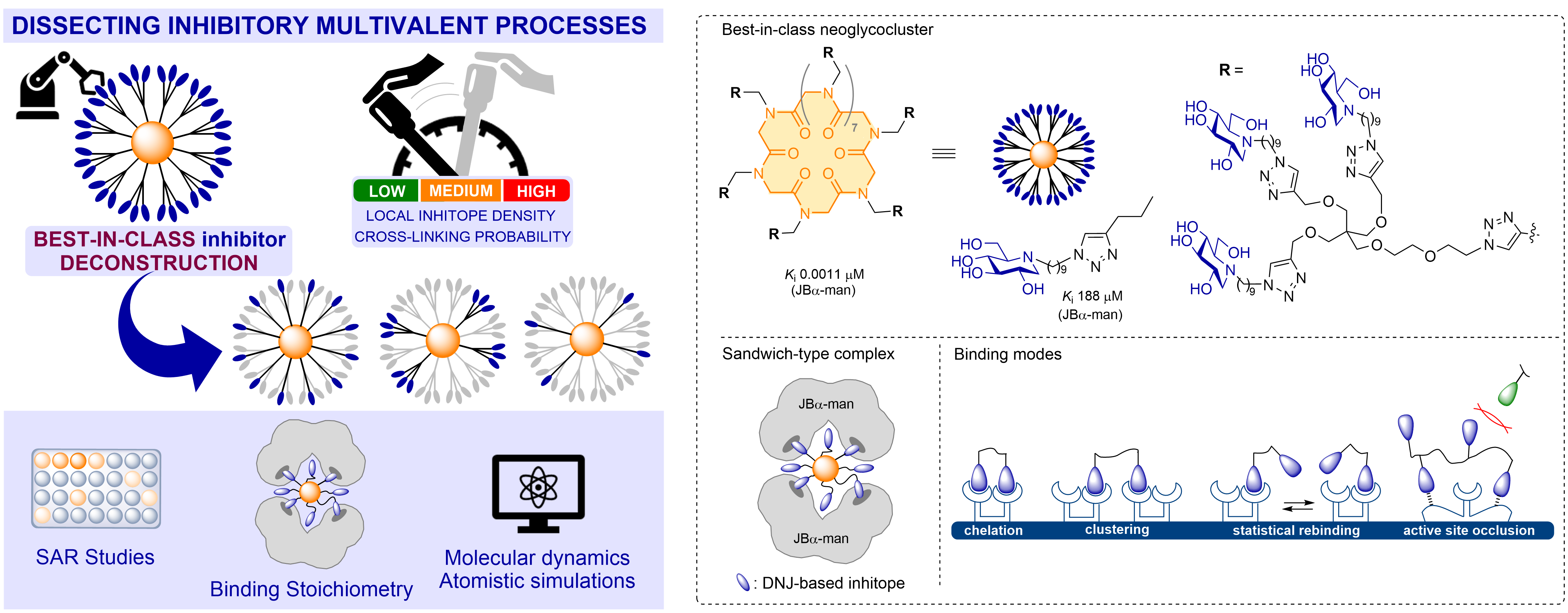
Deconstructing Best-in-Class Neoglycoclusters as a Tool for Dissecting Key Multivalent Processes in Glycosidase Inhibition
Chem. Eur. J. 2024, 30, e202304126.
Deconstructing for understanding. A structure-activity relationship study based on the top-down deconstruction of best-in-class multivalent inhibitors shed new lights on the contribution of chelation and statistical rebinding mechanisms underlying high multivalent effect in glycosidase inhibition. This experimental-theoretical approach provides insights from various dimensions (statistical, stoichiometric, electrostatics) and relevant guidelines for the design of optimized multivalent enzyme inhibitors.
From Sweet Molecular Giants to Square Sugars and vice versa
Synlett 2023, 34, 1866-1893.
An exciting journey. This account covers our research activities in the area of glycomimetics over roughly the last decade, from serendipity to serendipitose. Our efforts in understanding the structural basis of multivalent effects in glycosidase inhibition have led to decisive mechanistic insights and to the discovery of multimeric iminosugars displaying one of the largest binding enhancements reported so far for a non-polymeric enzyme inhibitor. Pushing the limits of the multivalent effect has also driven progress in synthetic methodology. Unexpected observation of side products en route to the synthesis of our targets has been the starting point of several new synthetic methodologies. We have also developed access to original square sugars and to carbohydrates with a quaternary (pseudo)anomeric position.
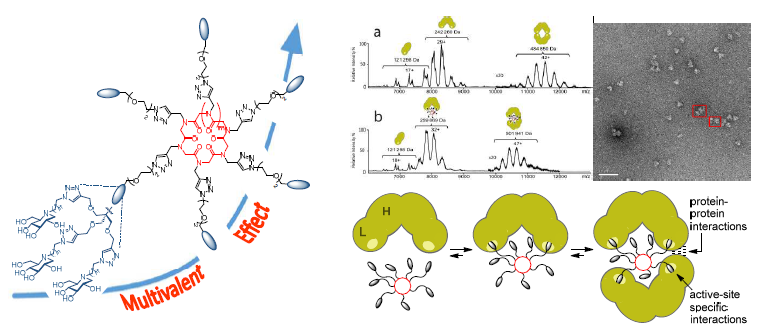
Iminosugar-Cyclopeptoid Conjugates Raise Multivalent Effect in Glycosidase Inhibition at Unprecedented High Levels
Chem. Eur. J. 2016, 22, 5151-5155.
Breaking sweet records! Cyclopeptoid-based iminosugar clusters show the largest multivalent effects ever reported in glycosidase inhibition with a binding enhancement up to 170 000 for Jack bean α-mannosidase (4700-fold enhancement per iminosugar unit!). Electron microscopy imaging and analytical data (MS, AUC-SV) support the formation of sandwich-type complexes in which two mannosidase molecules are cross-linked by one inhibitor.
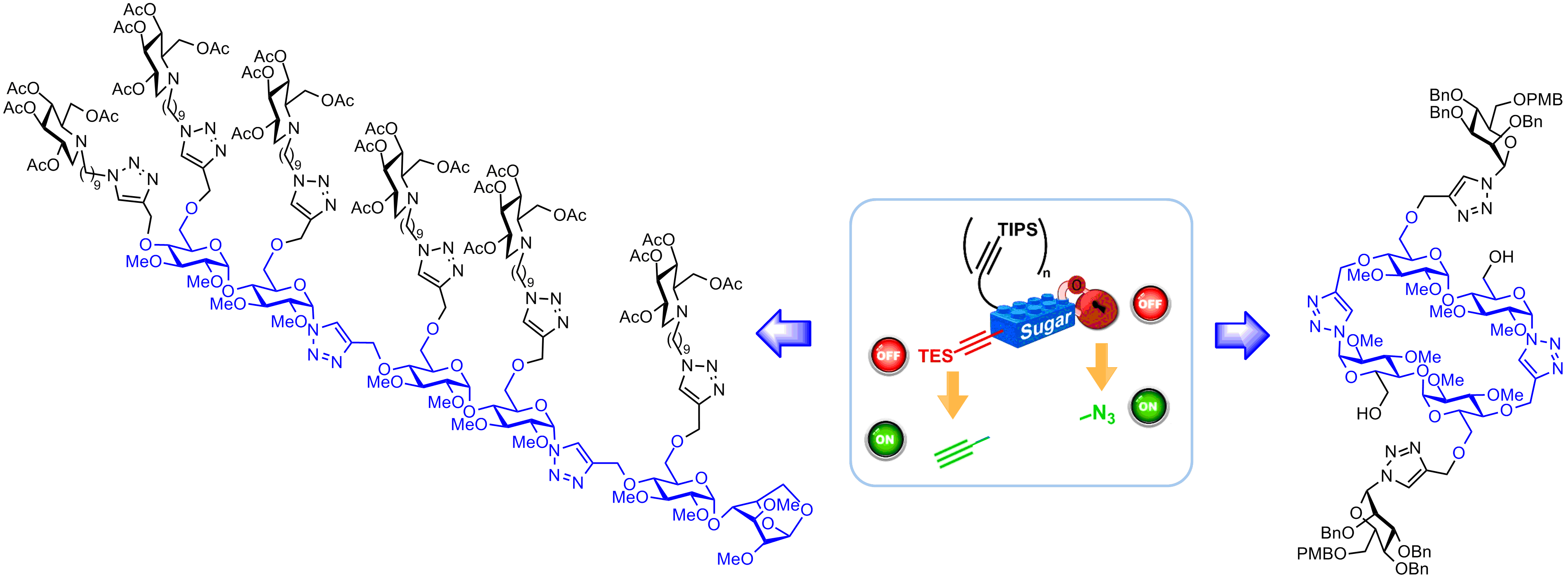
Towards a Molecular Lego Approach for the Diversity-oriented Synthesis of Cyclodextrin Analogues Designed as Scaffolds for Multivalent Systems
J. Org. Chem. 2015, 80, 10719.
Playing with sweet Lego® blocks. A new modular click strategy to access a diversity of cyclic and linear oligosaccharide analogues has been recently developed in our group. This unprecedented convergent approach is based on bifunctional sugar building blocks with two temporarily masked functionalities that can be orthogonally activated. The key steps of this molecular Lego® approach have been validated on a model disaccharide building block, leading to the first examples of new classes of glycoclusters and functionalized neo-(cyclic)oligosaccharides.
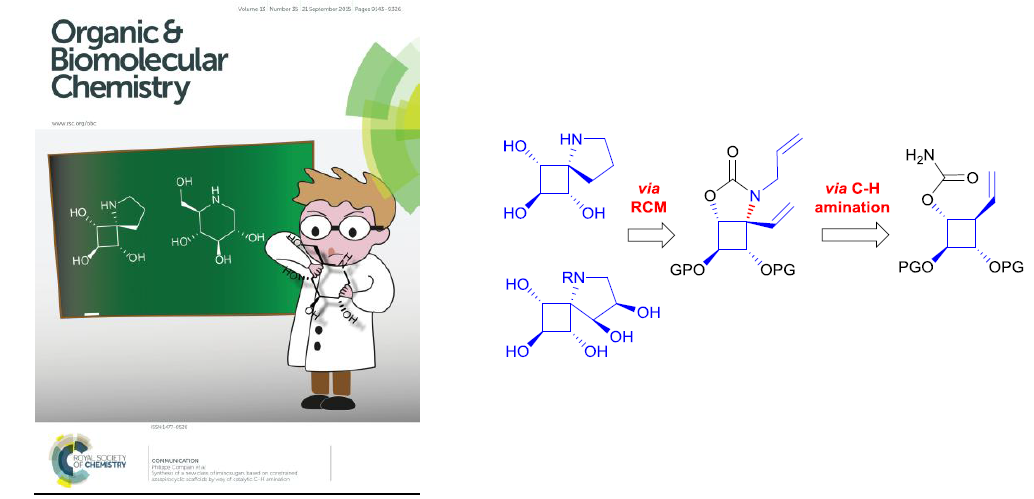
Synthesis of a new class of iminosugars based on constrained azaspirocyclic scaffolds by way of catalytic C-H amination
Org. Biomol. Chem. 2015, 13, 9176, Org. Biomol. Chem. 2016, 14, 2780-2796.
Twist it into a new design! The synthesis of the first examples of a new class of iminosugars based on constrained spirocyclic scaffolds has been achieved via Rh-catalyzed C(sp3)-H amination. In this process, the needed electronic control in securing high regioselectivity from substrates with a high density of activated C-H bonds was achieved by using a combination of activating and electron-withdrawing groups.
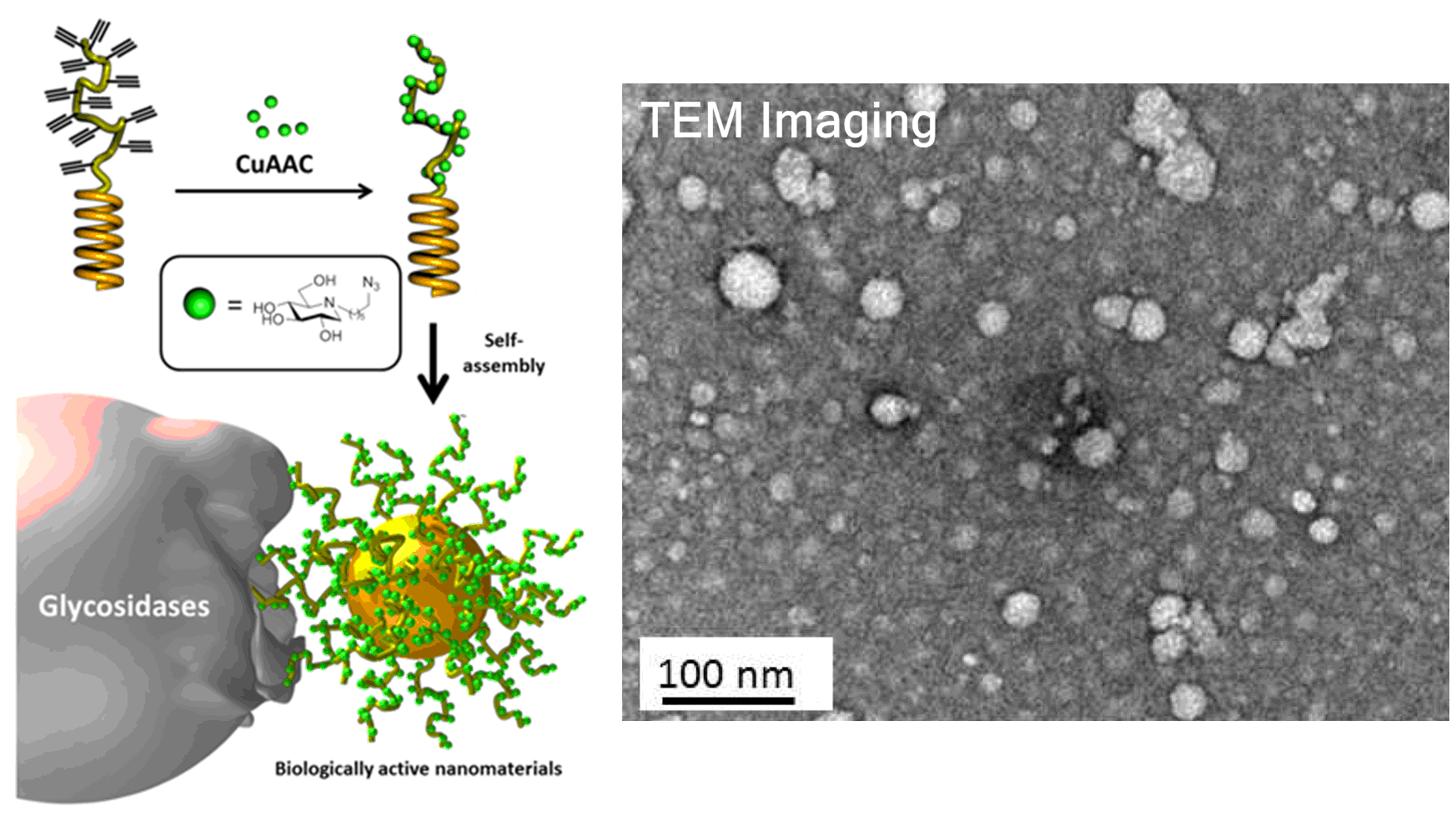
Iminosugar-based glycopolypeptides: glycosidase inhibition with bioinspired glycoprotein analogue micellar self-assemblies
Chem. Commun. 2014, 50, 3350.
Nano, sweet nano! The synthesis of the first examples of nanoparticles decorated with iminosugars has been recently performed in collaboration with the groups of Lecommandoux (Bordeaux), Heise (Dublin) and Ortiz Mellet (Seville). These biomimetic nanoparticles prepared by self-assembly of iminosugar-based glycopolypeptides evidenced remarkable multivalency properties when inhibiting α-mannosidase activity. This approach paves the way to biologically active drug delivery systems having glycosidase inhibition potency.
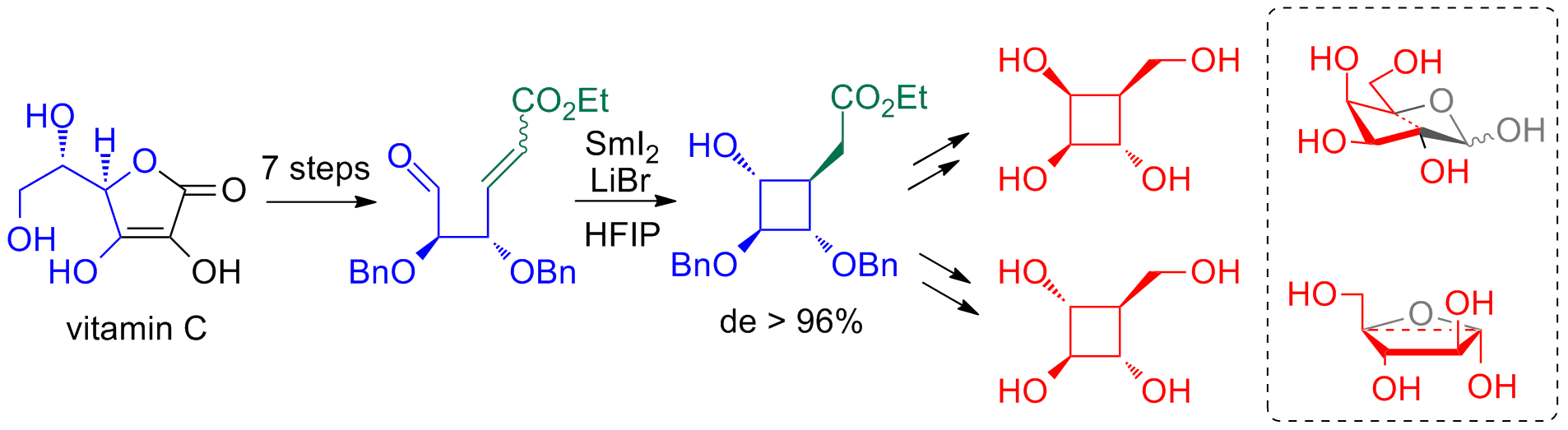
Synthesis of 4-Membered Carbasugars by way of Stereoselective SmI2-Mediated Aldehyde-alkene Cyclization
J. Org. Chem. 2013, 78, 6751.
From Vitamin C to 4-Membered Carbasugars. A stereodivergent synthesis of the first examples of 4-membered carbasugars has been achieved from vitamin C by way of an efficient intramolecular SmI2-mediated aldehyde-alkene coupling. In this key step, cylobutanes with four contiguous asymmetric centers are generated with a high level of stereocontrol.