|

|

|

|

|

|
Biological and Therapeutic targets
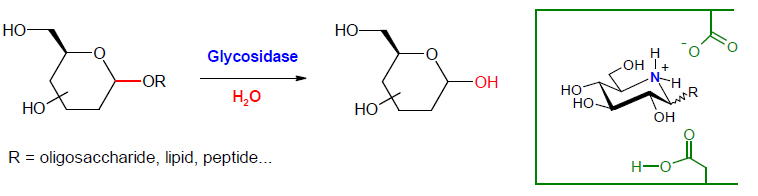
Biological targets. The enzymes targeted are mainly glycosidases and glycosyltransferases of therapeutic interest. Glycosidases and glycosyltransferases play a key role in numerous fundamental biological processes. These enzymes form the major catalytic machinery for the synthesis and hydrolysis of the glycosidic linkage. Glycosyltransferases are responsible for the synthesis of most glycoproteins and other glycoconjugates in mammalian system. They catalyze the transfer of a sugar moiety from an activated nucleotide sugar to the hydroxyl group of an acceptor which may be a growing oligosaccharide, a lipid or a protein. The second type of enzyme targeted, glycosidases, are found in essentially all domains of life, catalyzing the hydrolysis of the glycosidic linkage of oligosaccharides and glycoconjugates. This biologically widespread process is involved in the degradation of biomass, the trimming of cell- and viral-surface oligosaccharides and in the regulation of blood glucose levels. The high affinity of iminosugars for glycosidases is due to the fact that they are protonated in biological medium and thus interact strongly with an anionic group (carboxylate) in the enzyme active site.
Therapeutic targets. The wide scope of iminosugar biological activity as inhibitors of numerous enzymes such as glycosidase, glycosyltransferases, glycogen phosphorylases, nucleoside-processing enzymes, UDP-Galp mutase and metalloproteinases is breathtaking. As these enzymes are involved in numerous biological processes, iminosugars are now lead compounds for the treatment of an impressive variety of diseases including diabetes, cancers, viral infections, psoriasis and rare genetic diseases (lysosomal storage disorders and cystic fibrosis).
Our research projects are devoted to rare genetic diseases with a focus on lysosomal diseases and cystic fibrosis (mucoviscidosis). We have recently reported promising candidates for the development of an oral treatment against Gaucher disease, one of the most common lysosomal diseases. This rare hereditary disorder is due to the deficiency in a specific β-glucosidase involved in the catabolism of glycolipids. The disclosed iminosugars act as pharmacological chaperones and are able to double the residual cellular activity of the deficient glucosidase at nanomolar concentrations. We have also discovered recently potent submicromolar correctors of the protein involved in cystic fibrosis leading to the first description of a multivalent effect for correcting protein folding defects in cells (see below).
Selected publications
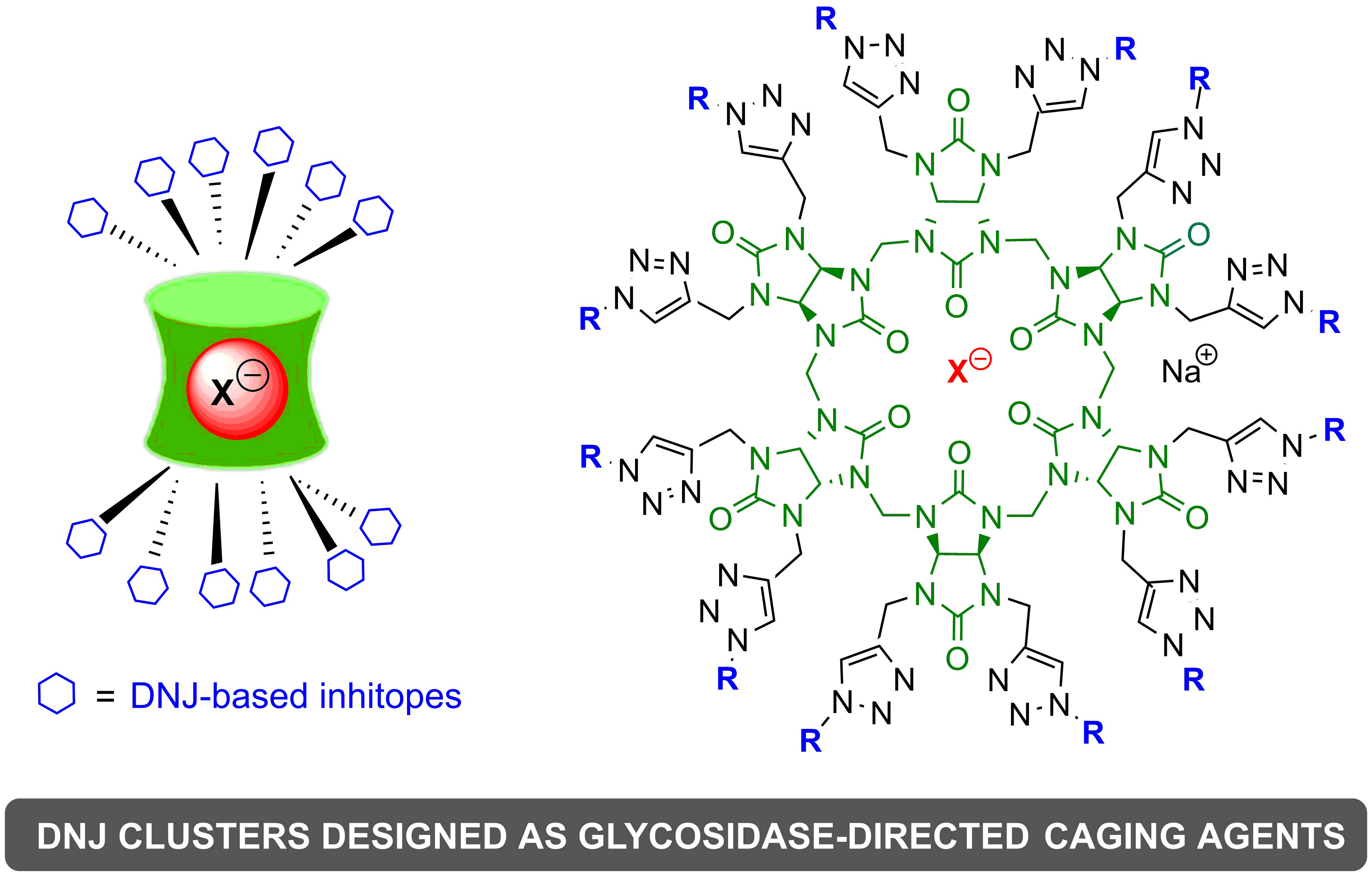
Bambus[4,6]urils As Dual Scaffolds for Multivalent Iminosugar Presentation and Ion Transport : Access to Unprecedented Glycosidase-directed Anion Caging Agents
Molecules 2022, 27, 4772.
Water-soluble glycosidase-directed anion caging agents. ABambusurils, BU[4] and BU[6], were used for the first time as multivalent scaffolds to link glycosidases inhibitors derived from 1-deoxynojirimycin (DNJ). DNJ and trivalent DNJ dendrons were grafted onto octapropargylated BU[4] and dodecapropargylated BU[6] using copper-catalyzed cycloaddition (CuAAC) to yield corresponding neoglycobambusurils bearing up to 24 iminosugar units. The inhibition potencies of neoglycobambusurils caging anions were evaluated against Jack Bean α-mannosidase and compared to monovalent DNJ derivatives. Strong affinity enhancement per inhibitory head were obtained for the clusters holding trivalent dendrons. Interestingly, the anion (bromide or iodide) encapsulated inside the cavity of BU[6] does not modify the inhibition potency of neoglycoBU[6] opening the way to water-soluble glycosidase-directed anion caging agents that may find applications in bio(in)organic chemistry or oncology.
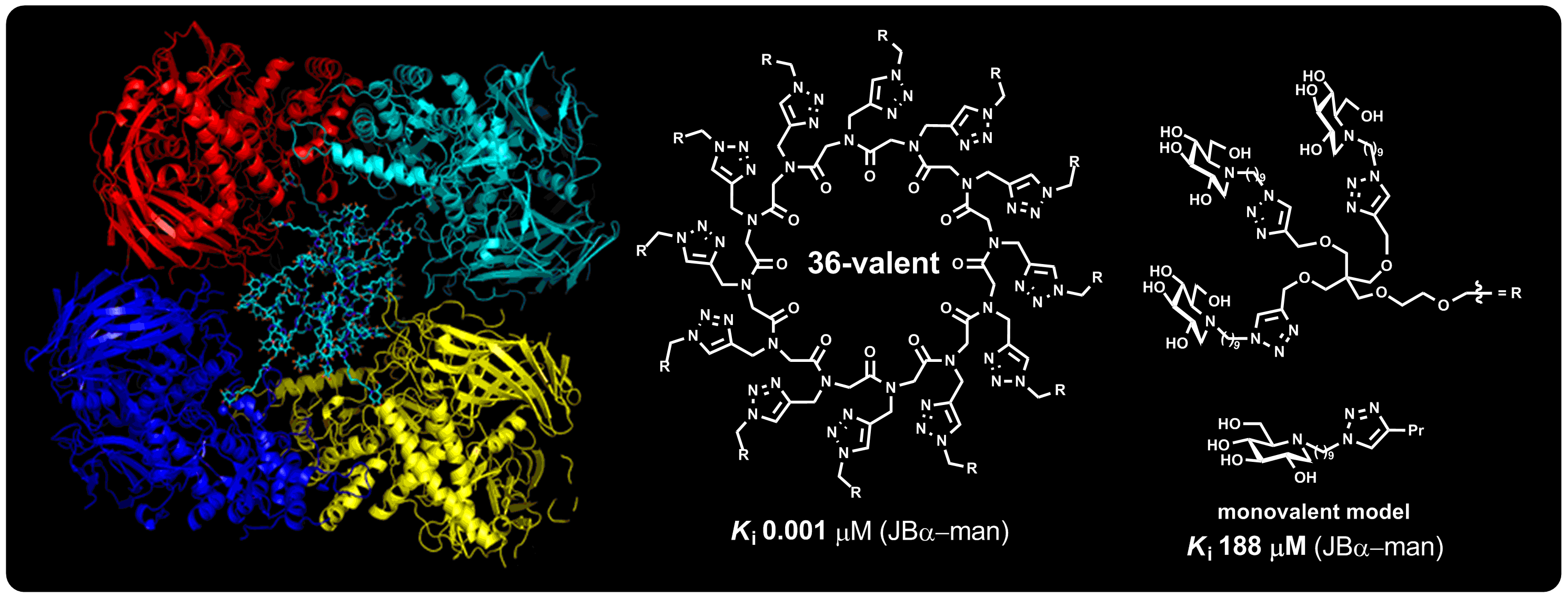
Structural Basis of Outstanding Multivalent Effects in Jack bean α-Mannosidase Inhibition
Angew. Chem. Int. Ed. 2018, 57, 8002.
Sweet sandwich! High-resolution crystal structures of Jack bean α-mannosidase (JBα-man) in apo and inhibited states provided decisive insight into the way a glycosidase and a multimeric inhibitor interact to produce outstanding multivalent inhibitory effects (see Chem. Eur. J. 2016, 22, 5151). Four ordered iminosugar heads of a 36-valent inhibitor (cyan in the picture) simultaneously engaged all four active sites of two dimeric JBα-man molecules to form a strong sandwich chelate complex. The enigma of the outstanding enhancements observed for the first time in 2010 in the inhibition of glycosidases is solved (Angew. Chem. Int. Ed. 2010, 49, 5753)!
Highlighted on the website of CNRS (click to see more details)
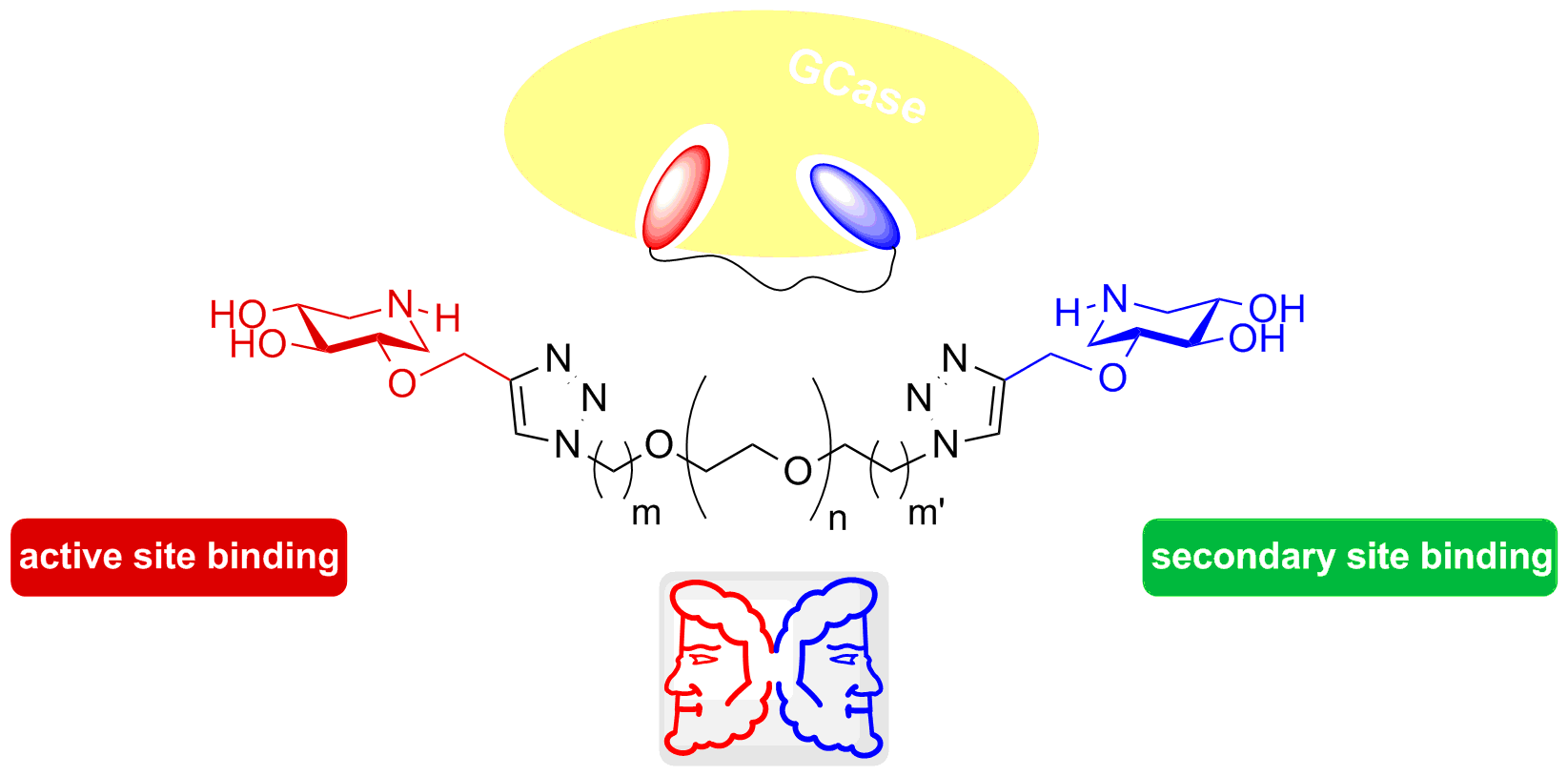
Stereodivergent Synthesis of Right- and Left-handed Iminoxylitol Heterodimers and Monomers. Study of Their Impact on β-Glucocerebrosidase Activity
Org. Biomol. Chem. 2017, 15, 3681.
Janus-type inhibitors. A library of dimers and heterodimers of both enantiomers of 2-O-alkylated iminoxylitol derivatives has been synthesised and evaluated on β-glucocerebrosidase (GCase), the enzyme responsible for Gaucher disease. The objective was to target simultaneously the active site and a secondary binding site of the glucosidase. All compounds having at least one (+)-2-O-alkyl iminoxylitol are GCase inhibitors in the nano molar range and are significant GCase activity enhancers in G202R fibroblats, as confirmed by a decrease of glucosylceramide levels and by co-localization studies.
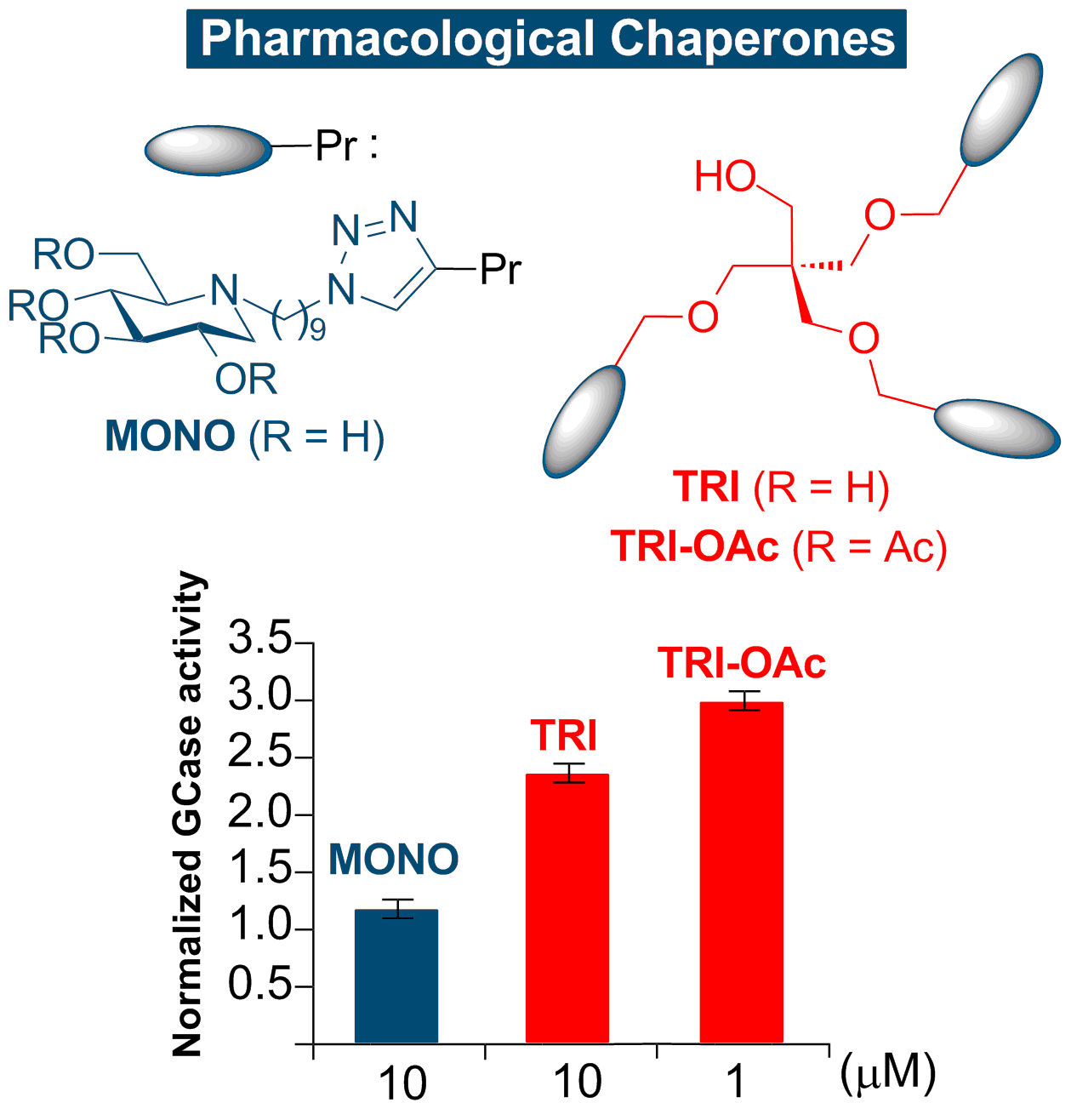
A Systematic Investigation of Iminosugar Click Clusters as Pharmacological Chaperones for the Treatment of Gaucher Disease
ChemBioChem 2014, 15, 309.
The right combination. Combining prodrug and multivalent strategies leads to an unprecedented pharmacological chaperone. A trivalent acetylated iminosugar is indeed able to increase mutant N370S β-glucocerebrosidase (GCase) activity levels by therapeutically relevant amounts as much as 3-fold in cells and at lower concentrations than the corresponding deprotected analogue.